
On Demand Conferences
Save on travel expenses, and learn on your own schedule.
ISPE’s On Demand offerings include recorded conference sessions, instructional lead courses and webinars to help you expand your skills and knowledge from the comfort of your desk.
ISPE Best of Pharma Series
Access the most premier presentations offering compelling insights, strategies, and best practices from experts representing global health authorities and leading pharma organizations.
Presentations are divided between four critical areas of pharma, allowing you to select the topic-specific package that is most pertinent to your knowledge needs:
2020 ISPE Asia Pacific Pharmaceutical Manufacturing Virtual Conference & Executive Forum On Demand Sessions
If you missed the 2020 ISPE Asia Pacific Pharmaceutical Manufacturing Virtual Conference & Executive Forum, this is your opportunity to gain access to all of the educational content from the conference and view it on your own schedule. This conference focused on emerging supply chain, quality, and compliance challenges in light of COVID-19. In addition, regulatory leaders shared best practices for assessing critical manufacturing processes and lessons learned to improve and sustain a reliable supply of quality medicine.
Here is a sneak peek of what you can expect to get from these on demand sessions. In this clip, program committee member Deva H. Puranam, Head of Global Quality Investigations, Mylan, reports out from Case Study 4 Drug Shortage Scenario: Managing an organization with multiple sites operating at different levels of quality systems maturity.
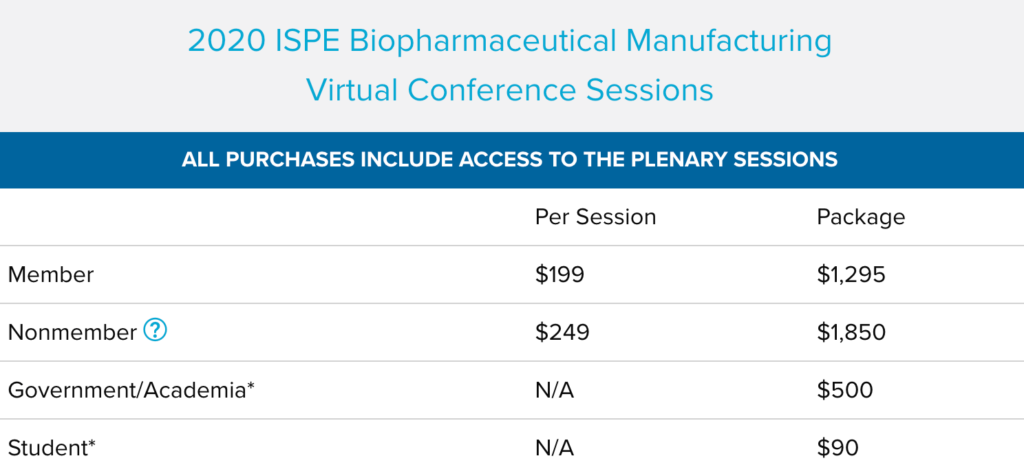

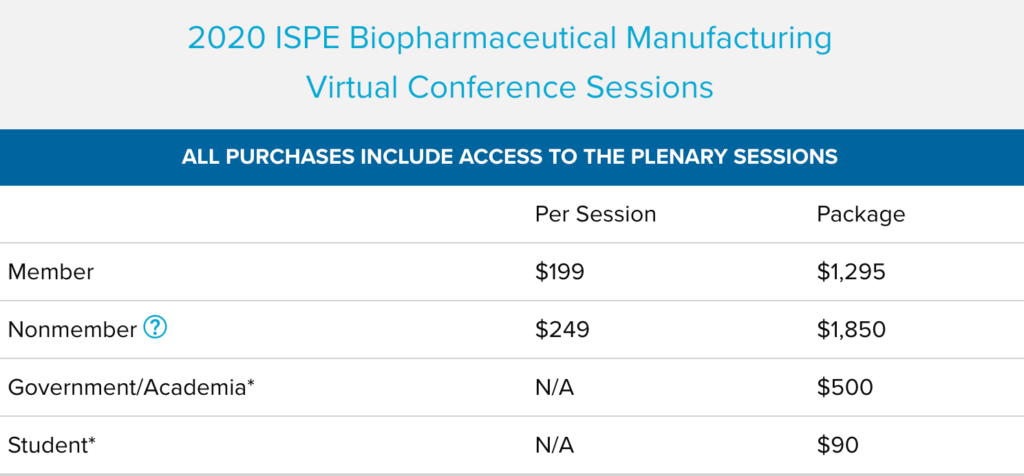
2020 ISPE Biopharmaceutical Manufacturing Virtual Conference Sessions

The 2020 ISPE Biopharmaceutical Manufacturing Virtual Conference focused on innovation in facilities, production methods, and technologies that enable a competitive and sustainable biopharmaceutical product supply for the future. This conference brought together experts that are developing, implementing, and operating advanced supply chains providing high quality medicines to global markets.
If you missed the conference, this is your opportunity to gain access to all of the educational content from the conference and view it on your own schedule. Get the biggest bang for your buck by purchasing the entire conference in one package or review the list of sessions to purchase individual.
Conference Sessions
Analytics Considerations for Continuous Manufacturing
Analytics Considerations for Continuous Manufacturing This session will be a series of presentations related to analytics and release assays critical to successful implementation of CM. The session will include perspectives on emerging approaches for assessment of quality attributes, on release testing, and on the importance of process control and PAT.
- Quality Considerations for the Multi-Attribute Method (MAM)
Sarah Rogstad, Staff Fellow, Chemist
FDA - Modern Microbiological Monitoring of Continuous Processing
Cheryl Essex, Head of Microbiological Control for Biologic Drugs
Sanofi - Integrating Analysis with Process Control for the Continuous Bioprocessing: Extending the Lifecycle Concept to Process Analytical Technologies
Jose C. Menezes, PhD, President and CEO
4Tune Engineering Ltd
Current Status on CM for Biologics: Development to Clinical
Speakers will provide insights on the current stage of deployment of CM for biological molecule clinical manufacturing from the point of view of process technologies, facility assets, and manufacturing operations. Examples of successes as well as challenges encountered will be shared.
- The Biopharmaceutical Industry Emerging Continuous and Integrated Platform for Recombinant Protein Jon Coffman, PhD, Senior Director of Bioprocess Technology and Engineering AstraZeneca
- Perspectives on the Development of Continuous Manufacturing for Biological Products
Mark Brower, Principal Scientist
Merck - Novel Technologies to Enable Continuous Manufacturing of Biologics
Govind Rao, PhD, Director, Center for Advanced Sensor Technology
University of Maryland, Baltimore County
Advances in Continuous Manufacturing for Small Molecule
Advances in Continuous Manufacturing for Small Molecule While continuous processes started with a few continuous steps, the maximal value of continuous manufacturing will be gained from increased integration. Several recent processes demonstrate this value. This session will focus on actual industrial processes, emphasizing the technical and business advantages of integration. Advances in the integration of unit operations, independently for API and drug product as well as the end-to-end integration of the two will be discussed over the course of the session.
- Full Integration of Continuous Manufacturing
Sal Mascia, PhD, President and CEO
CONTINUUS - Mighty Machines; Integrating Design of Chemical Processes and Flow Reactor Systems
Matthew M. Bio, PhD, President and CEO
Snapdragon Chemistry - Enabling End to End Continuous Manufacturing: FDA Perspective
Sharmista Chatterjee, PhD, Division Director
OPMA, OPQ/CDER
Emerging Technologies
Emerging Technologies The session brings together industrial case studies from practitioners who have delivered innovative solutions to enable the adoption of continuous processing within their companies.
- Commercializing New Chemical Technologies in Flow
Moiz Diwan, PhD, Director, Head of Enabling Technology Group
AbbVie - Continuous Processing for the Manufacture of Active Pharmaceutical Ingredients
Martin Johnson, PhD, Sr. Engineering Advisor
Eli Lilly & Company - The Use of Kinetic Modelling to Define a Design Space for a Continuous Process
Peter Shapland, PhD, Scientific Leader, R&D Medicinal Science & Technology
GSK Stevenage
Expert Roundtable
Expert Roundtable: How do we realize the full potential for Small Molecule in the new decade? Panel discussion with experts in the area of small molecule continuous manufacturing will discuss the technical, regulatory, and business hurdles for continuous manufacturing. This session will highlight CM as a continuum from E2E to hybrid approaches, including the need for “batch steps” for some products, requirement differences which can be a hindrance to global implementation of advanced technologies enabled by CM such as PAT, RTRT, and modeling; and why E2E CM may or may not be the optimal solution.
- Malcolm Berry, PhD, CEO & Founder MB
Chemistry Consulting, Ltd. - Sharmista Chatterjee, PhD, Division Director OPMA,
OPQ/CDER - Gabriella Dahlgren, PhD, Manager, Strategy Deployment
Janssen Supply Group LLC - Elizabeth Grieco, Director Vertex
- Michael O’Brien, PhD, Founder and President NGT
BioPharma Consultants
Advances in Continuous Manufacturing for Small Molecule
Advances in Continuous Manufacturing for Small Molecule While continuous processes started with a few continuous steps, the maximal value of continuous manufacturing will be gained from increased integration. Several recent processes demonstrate this value. This session will focus on actual industrial processes, emphasizing the technical and business advantages of integration. Advances in the integration of unit operations, independently for API and drug product as well as the end-to-end integration of the two will be discussed over the course of the session.
- Full Integration of Continuous Manufacturing
Sal Mascia, PhD, President and CEO
CONTINUUS - Mighty Machines; Integrating Design of Chemical Processes and Flow Reactor Systems
Matthew M. Bio, PhD, President and CEO
Snapdragon Chemistry - Enabling End to End Continuous Manufacturing: FDA Perspective
Sharmista Chatterjee, PhD, Division Director
OPMA, OPQ/CDER
Emerging Technologies
Emerging Technologies The session brings together industrial case studies from practitioners who have delivered innovative solutions to enable the adoption of continuous processing within their companies.
- Commercializing New Chemical Technologies in Flow
Moiz Diwan, PhD, Director, Head of Enabling Technology Group
AbbVie - Continuous Processing for the Manufacture of Active Pharmaceutical Ingredients
Martin Johnson, PhD, Sr. Engineering Advisor
Eli Lilly & Company - The Use of Kinetic Modelling to Define a Design Space for a Continuous Process
Peter Shapland, PhD, Scientific Leader, R&D Medicinal Science & Technology
GSK Stevenage
Expert Roundtable
Expert Roundtable: How do we realize the full potential for Small Molecule in the new decade? Panel discussion with experts in the area of small molecule continuous manufacturing will discuss the technical, regulatory, and business hurdles for continuous manufacturing. This session will highlight CM as a continuum from E2E to hybrid approaches, including the need for “batch steps” for some products, requirement differences which can be a hindrance to global implementation of advanced technologies enabled by CM such as PAT, RTRT, and modeling; and why E2E CM may or may not be the optimal solution.
- Malcolm Berry, PhD, CEO & Founder MB
Chemistry Consulting, Ltd. - Sharmista Chatterjee, PhD, Division Director OPMA,
OPQ/CDER - Gabriella Dahlgren, PhD, Manager, Strategy Deployment
Janssen Supply Group LLC - Elizabeth Grieco, Director Vertex
- Michael O’Brien, PhD, Founder and President NGT
BioPharma Consultants
*ISPE Membership is required for these rates.




